En tant qu’acteur spécialisé dans le domaine de la chirurgie orthopédique, nous sommes experts en chirurgie de la hanche et du sport. Notre savoir-faire se concentre sur la conception de prothèses orthopédiques qui améliorent la stabilité et la mobilité de chaque individu. Ainsi, comme la chirurgie, nous sommes persuadés que notre engagement en matière de responsabilité sociétale des entreprises participe au renforcement de nos relations avec nos partenaires, favorise la durabilité et participe au bien-être global de la société.
Politiques
S’ENGAGER, ENSEMBLE, POUR UN MONDE PLUS DURABLE

Société à taille humaine, nous intégrons de façon volontaire la RSE à notre stratégie. La politique RSE de DEDIENNE SANTÉ vise cinq grands objectifs pour permettre à notre activité de s’engager au plus près du développement durable :
- Contribuer aux enjeux du développement durable en intégrant la RSE dans notre stratégie et en assurant l’intégrité de l’entreprise ;
- Réduire notre empreinte environnementale en maîtrisant nos émissions de gaz à effet de serre, en gérant de manière efficace notre consommation de ressources, en améliorant la gestion de nos déchets et en préservant la biodiversité ;
- Garantir des produits responsables et de qualité en codéveloppant avec nos fournisseurs et partenaires un approvisionnement et un fret plus durable, en favorisant l’écoresponsabilité et l’écoconception et en assurant un service et des produits de qualité ;
- Améliorer la qualité de vie au travail de nos collaborateurs en permettant le développement des compétences, en préservant la santé et la sécurité de nos collaborateurs, en assurant des conditions de travail agréables et en s’engageant pour l’égalité et la diversité ;
- Être acteur de notre territoire en participant activement au développement du tissu de l’emploi local et en soutenant les causes sociales.

Afin d’assurer la réussite de nos projets RSE, les sujets sont traités au plus haut de la hiérarchie. Notre engagement est piloté par la direction mais également par notre comité RSE qui a pour vocation d’accompagner, de formaliser et de centraliser la démarche RSE impulsée par DEDIENNE SANTE.
Grâce à notre engagement en matière de RSE, DEDIENNE SANTE contribue à la réalisation de l’Agenda 2030 en travaillant à l’accomplissement des Objectifs de Développement Durable (ODD) établis par les Nations Unies. Nous sommes convaincus que cet engagement renforcera notre performance à long terme et contribuera à bâtir un monde meilleur et plus durable pour les générations futures.
MIEUX CONNAITRE SON IMPACT POUR MIEUX LE MAITRISER
Le saviez-vous ? 5°C c’est l’augmentation maximale de la température terrestre qui est attendue en 2100 en l’absence de l’adoption de mesures fortes.
Notre politique RSE vise à réduire notre empreinte environnementale grâce à la maîtrise de nos émissions de gaz à effet de serre. Conscient des enjeux climatiques, nous avons débuté l’aventure RSE par la réalisation d’un bilan carbone en 2023 avec l’aide de la BPI France, en partenariat avec l’ADEME et en collaboration avec l’ABC.
1920 T CO2 c’est le bilan de nos émissions de gaz à effet de serre chez DEDIENNE SANTE pour l’année fiscale 2022. Ce qui est équivalent :
- Aux émissions annuelles de 216 français ;
- A 190 tours de la Terre en voiture ;
- A la combustion de 605 680 litres de gazole.
Indicateurs clés :

Ces 1920 T de CO2 sont répartis de la façon suivante :
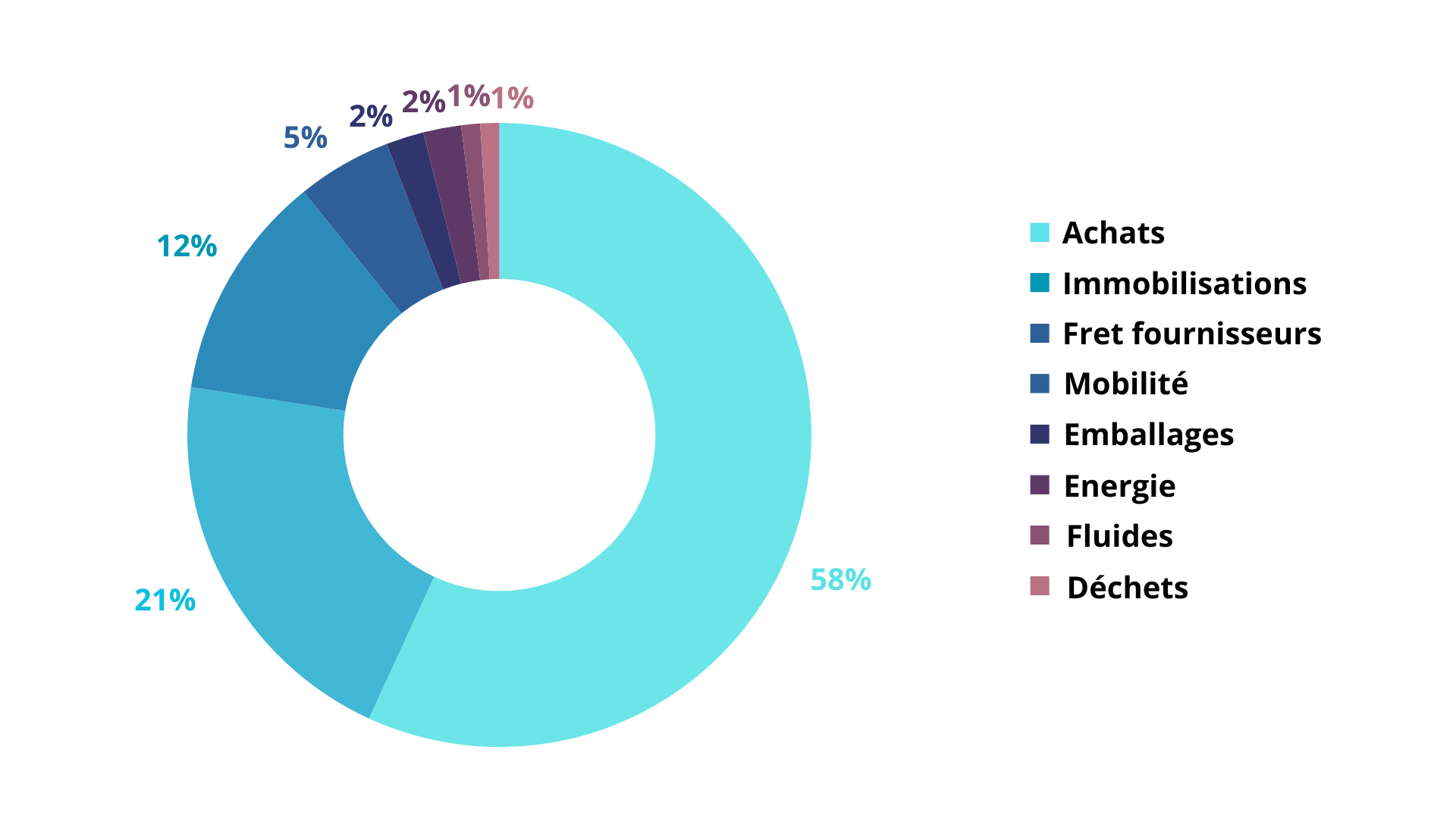
D’ici 2030, notre objectif est de réduire nos émissions de gaz à effet de serre, pour cela un plan d’action axée sur la décarbonation a été mis en place avec comme principales pistes d’actions : un travail sur l’approvisionnement des produits et un travail sur les immobilisations. Nous travaillons, ensemble, pour un monde meilleur et plus respectueux de l’environnement.
Pour accéder à notre Déclaration de Performance Extra-Financière, veuillez cliquer sur le bouton ci-dessous :
Pour accéder à notre Lettre d’engagement RSE, veuillez cliquer sur le bouton ci-dessous :
Pour plus d’informations sur nos engagements RSE, vous pouvez nous contacter via l’adresse électronique suivante : rse@dedienne-sante.com.


Dans un contexte où DEDIENNE SANTÉ s’affirme comme un acteur majeur de l’implantologie française, son orientation vers l’internationalisation prend une importance croissante. Cette expansion stratégique s’appuie sur un solide savoir-faire industriel et une ambition affirmée de rayonnement mondial, en mettant en avant les valeurs de qualité et de sécurité associées au label « Made in France ».
Miser sur l’international
DEDIENNE SANTÉ est aujourd’hui présente dans différents pays, Afrique, Amérique du Sud, Asie et Europe, réalisant ainsi une part significative de son chiffre d’affaires à l’export. Conscients des enjeux futurs et de la renommée dont bénéficient les industriels de l’implantologie française à travers le monde, notre ambition est d’étendre demain notre rayonnement à l’international en matière de chirurgie de la hanche, et du sport.
L’entreprise est particulièrement fière de ce parti-pris qui concentre les notions de savoir-faire français et de sécurité à valoriser au-delà des frontières. Notre site de fabrication est implanté depuis la création de DEDIENNE SANTÉ dans le Sud de la France, à proximité de la Métropole de Montpellier, réputée pour ses « pépites » sur le segment des Medtech.

Proposer des solutions adaptées au-delà de nos frontières
La politique de distribution de DEDIENNE SANTÉ à l’export vise à établir un partenariat avec des sociétés distributrices, très bien implantées localement et spécialisées dans l’orthopédie. DEDIENNE SANTÉ possède un savoir-faire industriel reconnu, que l’entreprise met au service du design et des professionnels de santé avec qui elle collabore pour satisfaire ses partenaires, au-delà de ses frontières.
Notre stratégie vise à proposer une offre structurée autour de gammes innovantes associées à un service totalement adapté. Nos interlocuteurs au Département commercial export sont appréciés pour leur réactivité et leur sens de l’écoute. Ils ont tous une parfaite connaissance de leur zone géographique et assurent formation et suivi de leurs clients. En partenariat avec le Département Marketing, un travail de fond a été initié pour réaliser des outils d’aide à la vente spécifiques à des zones géographiques bien ciblées.
Le département R&D chez DEDIENNE SANTÉ va vivre les mutations de sa profession liées au développement de l’entreprise et de l’orthopédie. Nous sommes vigilants à faire évoluer notre portefeuille de produits pour nous adapter rapidement à l’évolution de la demande et répondre à celle de nouveaux marchés.
Nos collaborateurs de la R&D bénéficient tous d’une connaissance aiguisée du marché de l’orthopédie et de ses produits en assurant une veille technologique permanente. Pour autant, ils sont l’interface entre la production et le département marketing. Nos recherches visent donc à optimiser technique, image, réalités économiques (s’assurer que le rapport qualité/prix des implants est adapté au marché) et pratiques commerciales en se conformant strictement aux instructions, aux règles et aux réglementations qui les régissent.
L’organisation du service est assurée en mode projet
Nos collaborateurs sont sélectionnés pour leur approche éthique et leurs qualités humaines que nous nous engageons à cultiver. Nos ingénieurs, techniciens et graphistes travaillent de concert avec les professionnels de santé et leurs équipes chirurgicales pour proposer des produits de très haute facture. Les relations qu’ils tissent ensemble sont fondées sur la confiance, le respect et une volonté commune d’entreprendre. En accompagnant les chirurgiens, nous garantissons ensemble aux patients les soins qu’ils recevront au cours de l’opération. Viser l’excellence afin d’aider les patients à renouer avec leurs habitudes de vie, avec une bonne vitalité physique…

Conscients des enjeux, le design produit constitue aujourd’hui un vaste champ d’expérimentation pour notre équipe R&D. L’ergonomie, plus précisément, constitue dorénavant le leitmotiv du service. Tout projet a pour objectif de conjuguer confort, sécurité et fiabilité pour garantir au chirurgien de disposer d’implants sûrs qui s’ajustent parfaitement avec la qualité de son intervention.
Chez DEDIENNE SANTÉ, nous sommes bien conscients que le confort recherché pour le praticien et ses équipes nécessite de pouvoir mettre en adéquation l’implant et l’ancillaire : manipuler facilement et avec la plus grande sécurité possible des produits particulièrement soignés et à forte valeur ajoutée. Nos produits et chacun des outils qui permettront la pose des implants font systématiquement l’objet de tests. Les implants subissent toutes les épreuves correctives nécessaires avant d’être soumis aux organismes d’accréditation qui seuls sont aptes à permettre leur commercialisation.
Le secteur médical est contraint par un cadre réglementaire fort et en constante évolution. Dans ce contexte, DEDIENNE Santé n’a de cesse d’investir dans ses processus Réglementaires et Qualité afin de maintenir l’innovation et d’améliorer la qualité et les performances de nos dispositifs.
Notre système de management de la qualité
En matière de prothèses orthopédiques et dans le domaine de la chirurgie du sport, le professionnalisme de DEDIENNE SANTÉ est reconnu. Parmi les valeurs que nous défendons, maintenir et optimiser notre Système de Management de la Qualité (SMQ) conforme aux exigences des clients et aux exigences réglementaires applicables en vigueur, constitue un facteur clé de succès. Notre démarche qualité, basée sur le référentiel EN ISO 13485 et sur la directive 93/42/CEE (relatifs aux dispositifs médicaux) garantit la conformité de nos produits aux exigences réglementaires des marchés sur lesquels nous les commercialisons.

Nous avons fait le choix d’être certifiés EN ISO 13485 par Kiwa Cermet Italia SpA, un des rares organismes à avoir été notifié pour le Règlement UE 2017/745.
Notre SMQ, constitué de procédures, instructions, méthodologies et pratiques documentées, repose sur une culture de la preuve permettant de garantir reproductibilité, amélioration continue et robustesse dans nos activités ainsi que l’accès permanent à leurs preuves de réalisation. In fine, notre SMQ a pour objectif de garantir la sécurité, les performances et la qualité des dispositifs fabriqués, et par conséquence de démontrer la conformité de nos produits aux exigences réglementaires des marchés sur lesquels ils sont commercialisés.
Nos certifications produits
Parmi les marchés sur lesquels nos dispositifs sont commercialisés, le marché Européen évolue dans un environnement réglementaire en pleine mutation : le Règlement (UE) 2017/745 est venu remplacer la Directive 93/42/CEE concernant les règles de mise à disposition sur le marché et de mise à service des dispositifs médicaux. Toutefois, une période transition voit cohabiter ancien et nouveau référentiel pour laisser aux différents acteurs le temps de s’organiser ; en tant que fabricant légal, DEDIENNE Santé est conforme aux conditions du Règlement (UE) 2017/745 en ce qui concerne ces dispositions transitoires.
Ainsi, nos certificats de conformité CE délivrés en vertu de la Directive 93/42/CEE bénéficient d’une prolongation de leur validité jusqu’en 2027 ou 2028 (selon la classe des dispositifs). L’ensemble de nos dossiers techniques ont été mis à jour pour intégrer les exigences du Règlement (UE) 2017/745 et sont en cours d’évaluation auprès d’un organisme notifié.
Une fois sur le marché, nos dispositifs font l’objet d’une surveillance assidue qui répond strictement aux obligations de vigilance (évolution de l’état de l’art, enquêtes de satisfaction client, réclamations client, incidents, mesures correctives de sécurité, tendances, données cliniques post-market) et nous permet d’optimiser notre organisation par le biais de corrections et d’actions correctives.
Servir la performance
Nous doter d’un haut degré d’exigence constitue les fondements mêmes de la philosophie DEDIENNE SANTÉ.
« Nous nous engageons à assurer la disponibilité de l’ensemble des ressources nécessaires pour mener à bien notre Politique Qualité, et déployer une démarche RSE avec comme objectif permanent le respect des exigences clients et réglementaires. »
Ludovic TOLEDO, D.G. DEDIENNE SANTÉ
En tant que concepteur, fabricant et distributeur d’implants orthopédiques, DEDIENNE SANTÉ concentre son attention sur la satisfaction de ses clients, en mobilisant l’engagement de chacun des collaborateurs de l’entreprise. Nous avons bien compris que l’essentiel de nos forces reposent sur la qualité de nos produits et les qualités humaines des collaborateurs. Ici plus que nulle part ailleurs, la fabrication de prothèse requiert des métiers très spécifiques. Nous sommes bien conscients que les compétences se font rares sur le marché.
En tant que société à taille humaine, pour faire la différence, nous nous faisons un devoir de donner du sens au travail et de favoriser la diversité. Nous proposons à chacun de nos collaborateurs un projet de développement professionnel personnalisé. Dans cette période délicate de renouveau, notre équipe RH se tient à la disposition des collaborateurs et de leur manager et ce, du recrutement, à l’intégration, au suivi et développement des compétences.
Ouverture d’esprit, sens de l’innovation, rigueur et sens du résultat
Outre les compétences techniques et la connaissance de nos marchés, nous valorisons la dimension humaine dans chacun de nos métiers : ouverture d’esprit, sens de l’innovation, rigueur et sens du résultat. La capacité à travailler en équipe est fortement valorisée. En parallèle, la mobilité est perçue chez DEDIENNE SANTÉ comme un vecteur de performance permettant de s’adapter et de rester compétitif mais nous pensons également qu’elle est source d’épanouissement pour nos collaborateurs. Pour cette raison, nous la plaçons au coeur de notre politique RH.
Nos métiers couvrent l’ensemble du cycle de développement du produit, de sa recherche à sa commercialisation. Dans le cadre de notre politique de développement, nous recherchons régulièrement des collaborateurs expérimentés dans divers domaines qui couvrent la Recherche et Développement, la production, la finance, les achats, la logistique et toutes fonctions support, les ventes, l’export, la communication et le marketing.
Conscient des enjeux et parce que nous apprécions l’implication, le regard nouveau et la curiosité des jeunes étudiants, nous proposons chaque année plusieurs offres de stages au sein des différents services.

En recherche active d’un poste ou d’un stage ?
Nous sommes à l’affut de talents pour renforcer nos équipes. Si un poste est susceptible d’être en adéquation avec votre profil, nous ne manquerons pas de vous contacter. Vous pouvez tout aussi bien nous adresser votre candidature spontanée et nous vous contacterons si une opportunité correspond à votre profil. Pour candidater, vous pouvez nous contacter via l’adresse électronique suivante : rh@dedienne-sante.com.
Dedienne Santé
Les fondations d’une marque française
Actualités
Notre blog
Partenaires
Mutualiser nos expériences
PROFESSIONNELS
SOCIÉTÉ
ACTUALITÉS
Fabriqué en France
